
How can an engine achieve its maximum efficiency? It must operate using reversible processes: a reversible process is one in which the system and the surroundings can be returned to state they were in before the process began. In reality there will be other losses (to friction, for example) that will reduce the efficiency. Note that this is the maximum possible efficiency for an engine. Work is just the input heat minus the rejected heat, so:

The following diagram is a representation of a heat engine, showing the energy flow:Īn important measure of a heat engine is its efficiency: how much of the input energy ends up doing useful work? The efficiency is calculated as a fraction (although it is often stated as a percentage):
Describe the second law of thermodynamics full#
In a full cycle of a heat engine, three things happen: At one stage the system is heated, at another it is cooled. A cycle of heating and cooling will move the piston up and down.Ī necessary component of a heat engine, then, is that two temperatures are involved.
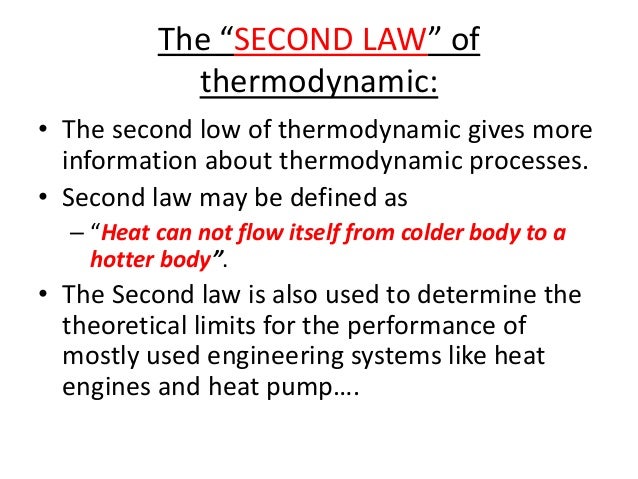
Once the gas is heated, moving the piston up, it can be cooled and the piston will move back down. A practical engine goes through cycles the piston has to move back and forth. This wouldn't be a particularly practical engine, though, because once the gas reaches equilibrium the motion would stop. If the gas is heated, it expands, moving the piston. A basic heat engine consists of a gas confined by a piston in a cylinder. We'll move on to look at heat engines, which are devices that use heat to do work. On the other hand, heat flows from hot to cold spontaneously. Heat can be made to flow from a colder region to a hotter region, which is exactly what happens in an air conditioner, but heat only does this when it is forced. The second law states that heat flows naturally from regions of higher temperature to regions of lower temperature, but that it will not flow naturally the other way. The second law of thermodynamics comes in more than one form, but let's state in a way that makes it obviously true, based on what you've observed from simply being alive. Heat engines and the second law Heat engines and the second law


 0 kommentar(er)
0 kommentar(er)
